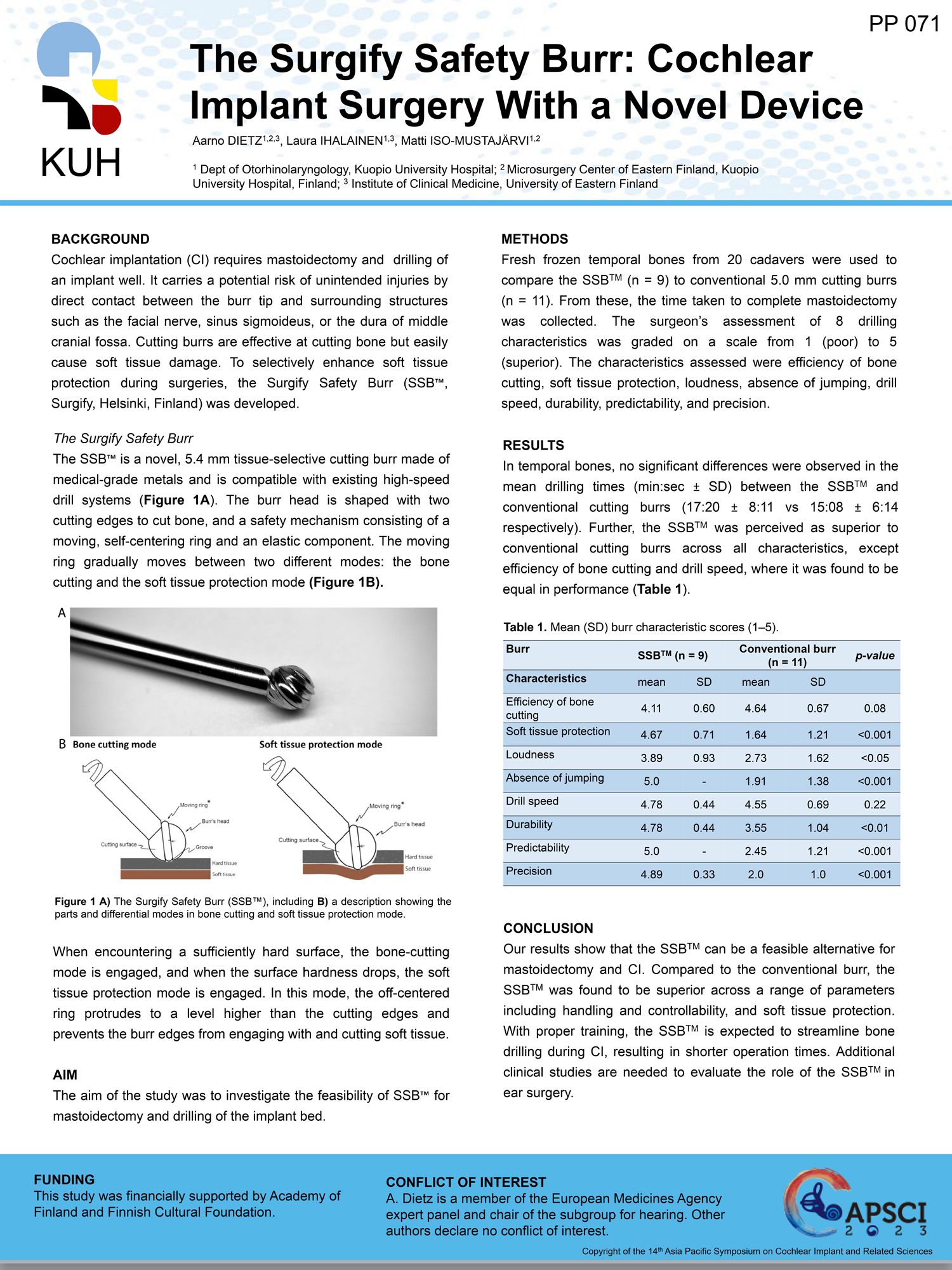
Surgify Halo™is a CE-marked medical device approved for clinical use in the EU (Notified Body no. 0537).
We are working diligently to acquire regulatory approvals in additional geographies
The safety and efficacy of Surgify Halo™ in bone removal procedures has been extensively tested in laboratory environments as well as in pre-clinical and clinical trials.
Presented data
Published data

Results from pre-clinical testing
In pre-clinical testing Surgify Halo™ was evaluated in different bone specimens (human and animal bone) by eight neuro and spine surgeons from three university hospitals across Finland. A total of 77 surgeries were performed in these tests, evaluating the soft-tissue protecting features of Surgify Halo™ compared with conventional cutting burrs.
In these tests soft-tissue injuries occurred in none (0%) of the performed surgeries with Surgify Halo™ and in 10 (29%) of the performed surgeries with conventional burrs.